Carbohydrates : Biochemistry and Clinical Pathology
The normal fasting blood sugar level range is typically: ☐ 70 -100 mg/dl
Here’s why the other options are incorrect for normal fasting blood sugar levels:
70 – 140 mg/dl: While 70 mg/dl is within the normal range, 140 mg/dl is higher than normal. Fasting blood sugar levels consistently above 100 mg/dl but below 126 mg/dl can indicate prediabetes or insulin resistance.
100 – 125 mg/dl: This range is considered to be indicative of prediabetes rather than normal fasting blood sugar levels. A fasting blood sugar level consistently in this range suggests that someone may be at higher risk for developing diabetes.
100 – 140 mg/dl : This range is also above the normal fasting blood sugar range. Similar to the previous option, levels above 100 mg/dl are indicative of a higher risk for diabetes and are not considered normal fasting levels.
The caloric value of carbohydrates is approximately: ☐ 4 Kcal/gm
Carbohydrates provide about 4 kilocalories (Kcal) per gram. This is a standard value used in nutrition to estimate energy content.
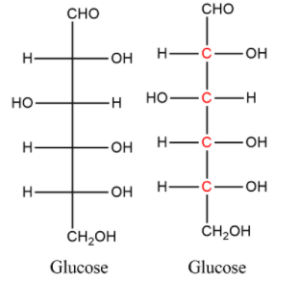
The number of asymmetric (chiral) carbon atoms in glucose is: ☐ 4
Glucose has four asymmetric carbon atoms at carbons 4, 5, and 6 in the molecule. These chiral centers are responsible for glucose’s ability to exist in different optical isomers.
The option that acts like a fuel in driving the body is: ☐ Carbohydrates
Carbohydrates are a primary source of energy for the body. They are broken down into glucose, which cells use for energy.
While fats also provide energy and vitamins are essential for various metabolic processes, carbohydrates are the most immediate and readily available fuel source for the body’s needs.
The major function of carbohydrates is: ☐ storage of energy
Carbohydrates are primarily used by the body as a source of energy. They are broken down into glucose, which is used to fuel various physiological processes.
While carbohydrates also play roles in other functions, such as influencing water balance indirectly and being involved in some metabolic pathways, their primary role is to provide and store energy.
The percentage of total calories obtained from carbohydrates should be between: ☐ 50% to 60%
This range is generally recommended by dietary guidelines for a balanced diet, as carbohydrates are a primary source of energy.
The other options are too low to meet the body’s energy needs effectively.
A monosaccharide is: ☐ Ribose
Ribose is a simple sugar (monosaccharide) with a single sugar unit.
In contrast:
Lactose is a disaccharide composed of glucose and galactose.
Sucrose is a disaccharide composed of glucose and fructose.
Maltose is a disaccharide composed of two glucose units.
Galactose is a: ☐ Monosaccharide
Galactose is a single sugar unit and a type of simple sugar. It is one of the basic building blocks of more complex carbohydrates.
In contrast:
Disaccharide refers to sugars composed of two monosaccharide units, like lactose or sucrose.
Polysaccharide refers to long chains of monosaccharide units, like starch or cellulose.
Sucrose is a disaccharide composed of glucose and fructose.
The correct answer is a mixture of Mannitol and Sorbitol.
D-Fructose, a simple sugar, can be reduced to form a mixture of two sugar alcohols: Mannitol and Sorbitol. This reduction reaction involves the addition of hydrogen to the carbonyl group (aldehyde or ketone) in the sugar molecule, resulting in the formation of a hydroxyl group (-OH).
The reduction of D-Fructose yields:
– Mannitol (about 50-60%): a straight-chain sugar alcohol
– Sorbitol (about 40-50%): a straight-chain sugar alcohol
So, the correct answer is a mixture of Mannitol and Sorbitol.
The preferred substrate for hexokinase is glucose.
Hexokinase is an enzyme that catalyzes the phosphorylation of glucose to glucose-6-phosphate in the first step of glycolysis.
While hexokinase can also phosphorylate other sugars like fructose, glucose is its primary and preferred substrate due to its central role in energy production and metabolism.
The sugar found in milk is lactose, which is a disaccharide composed of glucose and galactose.
So, both galactose and glucose are components of lactose, but if you have to choose one of the options given, galactose is more specific to the sugar found in milk.
The sugar known as “milk sugar” is lactose. Lactose is the disaccharide found in milk and dairy products, consisting of glucose and galactose.
The correct answer is (d) All of these.
Carbohydrates, which include sugars, starches, and fibers, can contain various functional groups, including:
Alcoholic hydroxyl groups (-OH): These are present in all carbohydrates, allowing them to form hydrogen bonds and engage in various chemical reactions.
Aldehyde group (-CHO): This functional group is found in aldoses, a type of monosaccharide (simple sugar), such as glucose and galactose.
Ketone group (-C=O): This functional group is present in ketoses, another type of monosaccharide, such as fructose and ribulose.
The correct answer is (a) Type-1 diabetes.
Type 1 diabetes, also known as insulin-dependent diabetes mellitus (IDDM), is a chronic autoimmune disease in which the body’s immune system attacks and destroys the insulin-producing beta cells in the pancreas. As a result, people with type 1 diabetes are unable to produce enough insulin and require insulin injections or an insulin pump to control their blood sugar levels.
Type 2 diabetes, on the other hand, is a metabolic disorder characterized by insulin resistance (when the body’s cells become less responsive to insulin) and impaired insulin secretion. While people with type 2 diabetes may eventually require insulin therapy, it is not always insulin-dependent.
The correct answer is Galactose-1-phosphate uridyl transferase.
Galactosemia is a rare genetic disorder caused by the deficiency of the enzyme galactose-1-phosphate uridyl transferase (GALT). This enzyme is necessary for the conversion of galactose, a sugar found in milk and other dairy products, into glucose, which can be used by the body for energy.
The deficiency of GALT leads to the accumulation of galactose-1-phosphate, causing damage to various organs, including the liver, kidneys, and brain.
The other options are incorrect because:
– Glucose-6-phosphatase: This enzyme is involved in glycogenolysis and gluconeogenesis, but its deficiency does not cause galactosemia.
– HMG CoA reductase: This enzyme is involved in cholesterol synthesis, and its deficiency does not cause galactosemia.
– L-Gulonolactone oxidase: This enzyme is involved in the synthesis of ascorbic acid (vitamin C), and its deficiency does not cause galactosemia.
Note: There are other forms of galactosemia caused by deficiencies of other enzymes, such as galactokinase and UDP-galactose-4-epimerase, but GALT deficiency is the most common cause.
The correct answer is Decrease production of Insulin.
Insulin-dependent diabetes mellitus (IDDM), also known as Type 1 diabetes, is caused by a deficiency in insulin production due to the autoimmune destruction of the insulin-producing beta cells in the pancreas.
This results in:
- Decreased insulin production
- Increased blood glucose levels
- Dependence on exogenous insulin (insulin injections or an insulin pump) to control blood sugar levels
The other options are incorrect because:
– Increase production of Insulin: This is not a cause of IDDM; in fact, the opposite occurs.
– Decrease production of glucagon: Glucagon is a hormone that raises blood glucose levels. Decreased glucagon production would not cause IDDM.
– Increase production of glucagon: While glucagon levels may be elevated in IDDM due to the lack of insulin, it is not the primary cause of the disease.
Note: In Type 1 diabetes, the pancreas produces little to no insulin, whereas in Type 2 diabetes, the body becomes resistant to insulin, and the pancreas may initially produce more insulin to compensate, but eventually, insulin production may also decrease.
The correct answer is Sucrose.
Benedict’s test is a chemical test used to detect the presence of reducing sugars, such as monosaccharides and some disaccharides. A positive Benedict’s test is indicated by a color change to orange, yellow, or red, depending on the concentration of the reducing sugar.
Sucrose, a disaccharide composed of glucose and fructose, does not react with Benedict’s solution because it is not a reducing sugar. This is because the aldehyde or ketone group, necessary for the reaction, is involved in the glycosidic bond between the glucose and fructose molecules.
The other options are incorrect because:
- Lactose: A disaccharide composed of glucose and galactose, which gives a positive Benedict’s test.
- Maltose: A disaccharide composed of two glucose molecules, which gives a positive Benedict’s test.
- Glucose: A monosaccharide that gives a positive Benedict’s test.
Note: Benedict’s test is commonly used to detect the presence of reducing sugars in urine, which can be an indicator of diabetes or other metabolic disorders.
The correct answer is Molisch’s Test.
Molisch’s test is a general test used to detect the presence of carbohydrates, including monosaccharides, disaccharides, and polysaccharides. It involves the reaction of carbohydrates with sulfuric acid and alpha-naphthol, resulting in a purple-colored compound.
The other options are specific tests for different types of compounds:
Mayer’s Test: Used to detect alkaloids.
Benedict’s Test: Used to detect reducing sugars (monosaccharides and some disaccharides).
Shinoda Test: Used to detect flavonoids.
Molisch’s test is a general test for carbohydrates because it detects the presence of any carbohydrate-containing compound, whereas the other tests are more specific.
Here’s a brief overview of each test:
– Molisch’s Test: Carbohydrates → purple color
– Benedict’s Test: Reducing sugars → orange/yellow/red color
– Mayer’s Test: Alkaloids → white precipitate
– Shinoda Test: Flavonoids → red color
The correct answer is Cherry red color.
Seliwanoff’s test is a chemical test used to detect the presence of fructose (a ketohexose) and other ketoses.
The test involves heating a sample with Seliwanoff’s reagent (resorcinol and hydrochloric acid). Fructose reacts with the reagent to produce a cherry red color.
Fructose Resorcinol HCl → Cherry red color
The other options are incorrect because:
Deep Blue color: Not associated with Seliwanoff’s test for fructose.
White color precipitate: Typically associated with tests for alkaloids or proteins.
Green color precipitate: Not associated with Seliwanoff’s test for fructose.
Seliwanoff’s test is specific for fructose and other ketoses, distinguishing them from aldoses (like glucose) which do not react with the reagent.
